To all Support, Inc. staff, providers, and families;
As the COVID-19 pandemic has drastically changed our lives over the last 9 months, there has been a lot of talk, questions and hopes for a COVID-19 vaccination. An FDA (Food and Drug Administration) advisory committee met on Thursday December 10th to review the Pfizer vaccine. The committee resulted in a 77% “yes” vote that based on the totality of evidence, the benefit of the Pfizer vaccine outweighs the risk for use in individuals age 16 and older. This was the last step in the process for Pfizer before the FDA approves emergency use of their COVID-19 vaccine. Over the weekend, the FDA accepted the committee’s recommendation and approved the vaccine under the Emergency Use Authorization. His is a pivotal moment in the pandemic as the first vaccine being approved for use in the United States. While we do not have all the answers, we wanted to provide some information on what we do know at this point.
Q: What is an Emergency Use Authorization?
A: An EUA (Emergency Use Authorization) is a way to facilitate the availability and use of things like vaccines during public health emergencies such as the COVID-19 pandemic. Under EUA the FDA may allow an unapproved medical product to prevent serious life-threatening diseases or conditions. Manufacturers submit an EUA request to the FDA to be evaluated and to determine if statutory criteria are met. You can read more about Emergency use Authorizations here.
Q: When can I get the vaccine?
A: This week, Colorado released a 3 phased roll out plan for the vaccine. Below is a description and timeline for each phase.
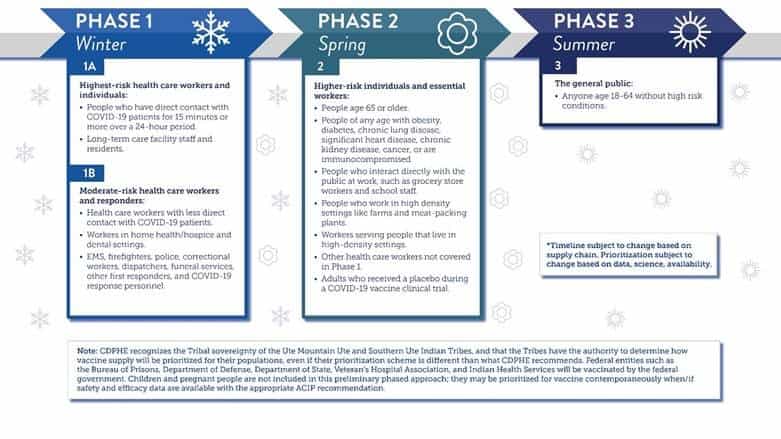
Q: Where can I get the vaccine?
A: Once a vaccine has been approved for use by the FDA, Phase 1 recipients will receive the vaccine through their employer (such as hospitals), local health agencies or through the Federal Government’s pharmacy partnership for long term care programs. The Pfizer vaccine requires ulta low temperature storage (-60 to -80 Celsius). Colorado has identified 24 locations with the ability to store the vaccine at this temperature and has purchased an additional 10 ulta low temperature freezers. The state will release information for phase 2 and phase 3 vaccination locations in the future.
Q: Will there only be one vaccine option?
A: At this time, the Pfizer vaccine has been approved. Moderna has also applied to the FDA for authorization through the Emergency use Authorization. There are 3 additional companies working on clinical trials for a vaccination. It is likely individual states and counties will get different vaccines based on manufacturing and distribution from each company.
News of a vaccine is encouraging. At the moment constituents of Support, Inc. may be eligible to receive the vaccine during Phase 2. This may change and as we learn more information, we will send additional updates. While we all anticipate the vaccine, it continues to be important we all take steps to keep ourselves, our families and individual we support safe. This includes, wearing a face covering/ mask when we are in public, washing hands with soap and water or using hand sanitizer frequently, minimizing contact with people we don’t live with and when we do have to have contact with people who don’t live in our home, staying 6 feet apart.
If you have any questions, please don’t hesitate to contact Laura at Laura.Viers@supportinc.com